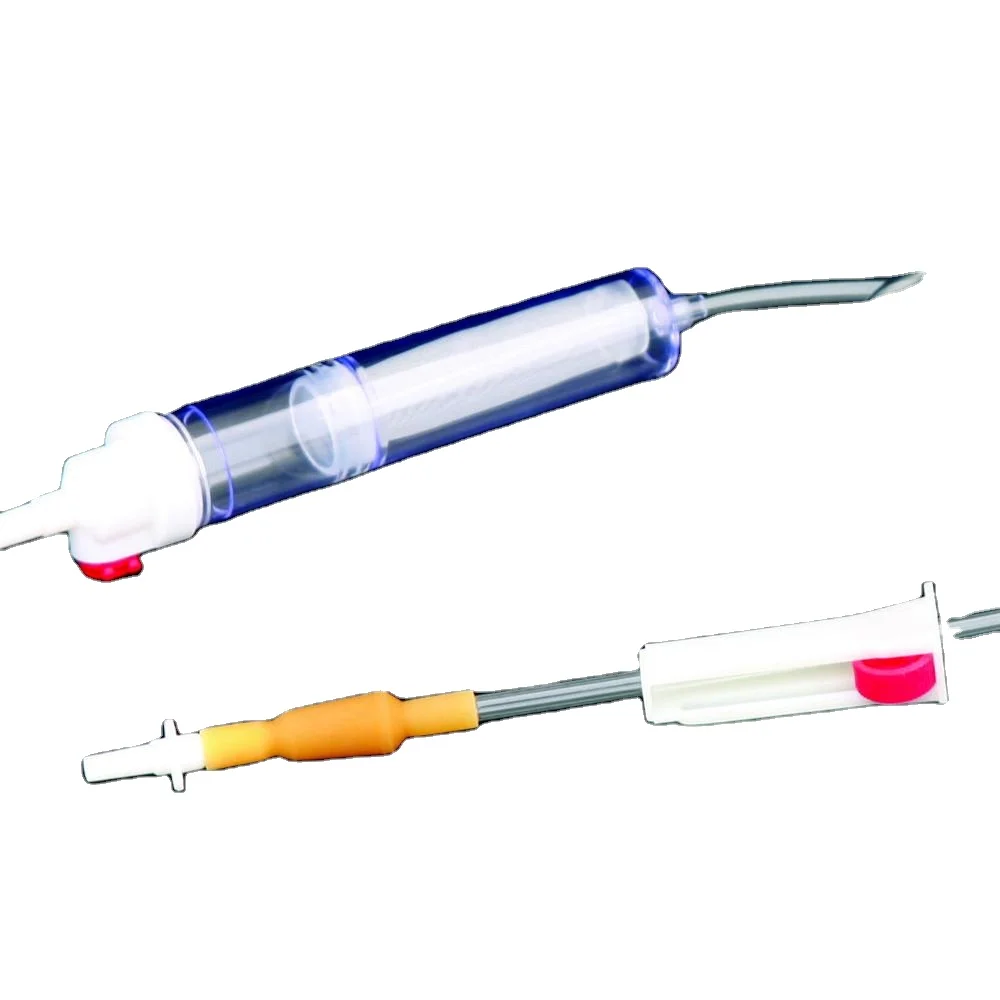
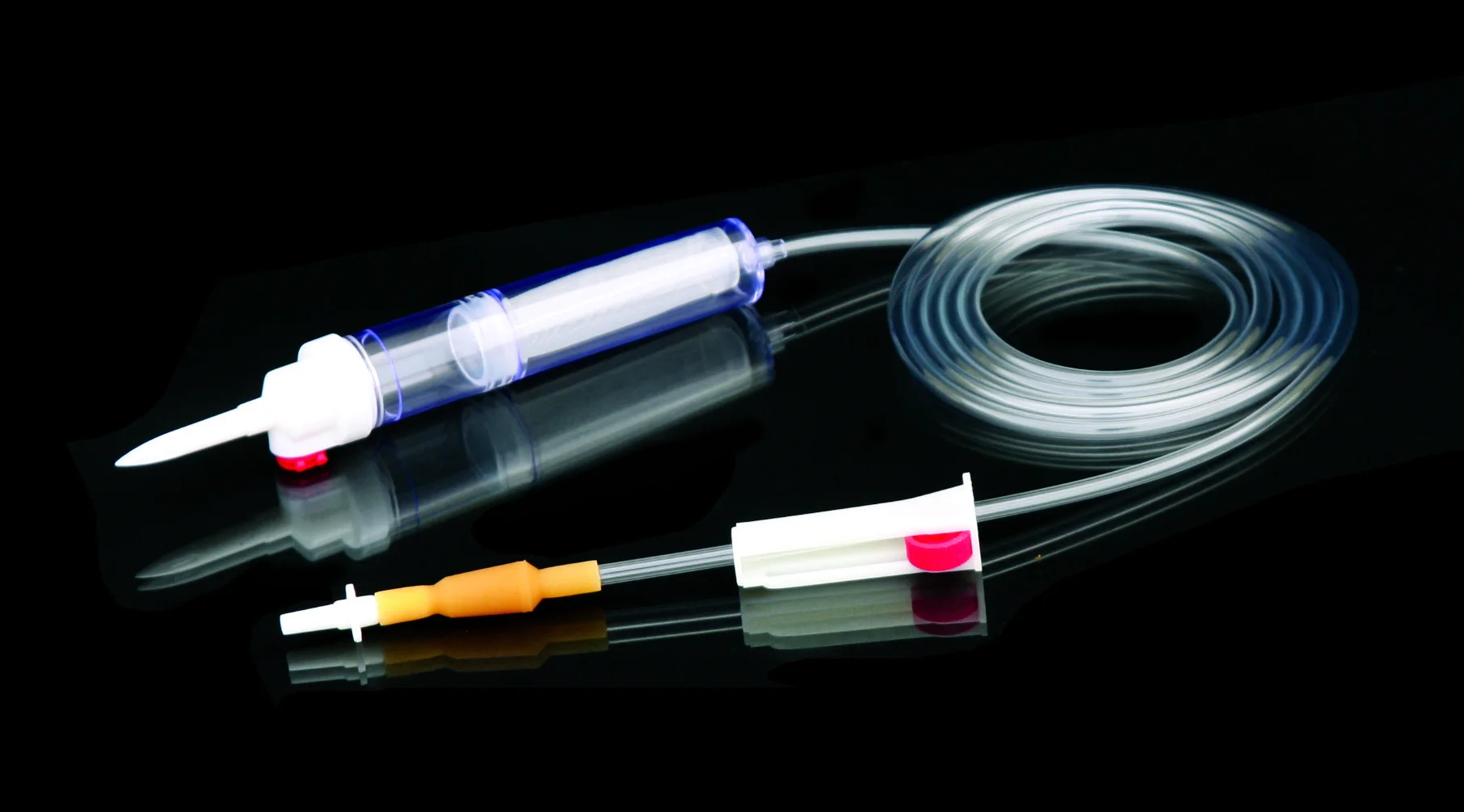
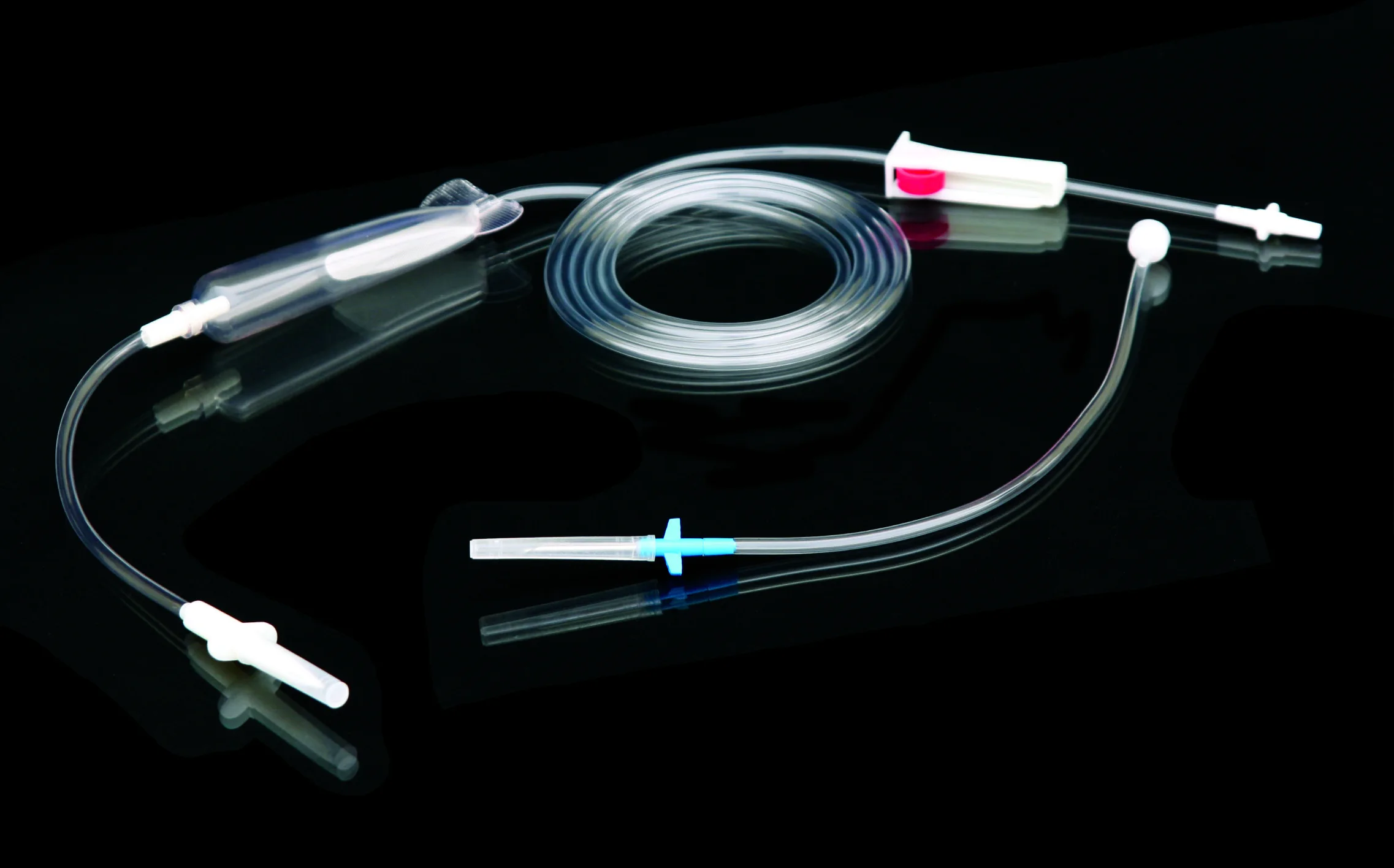
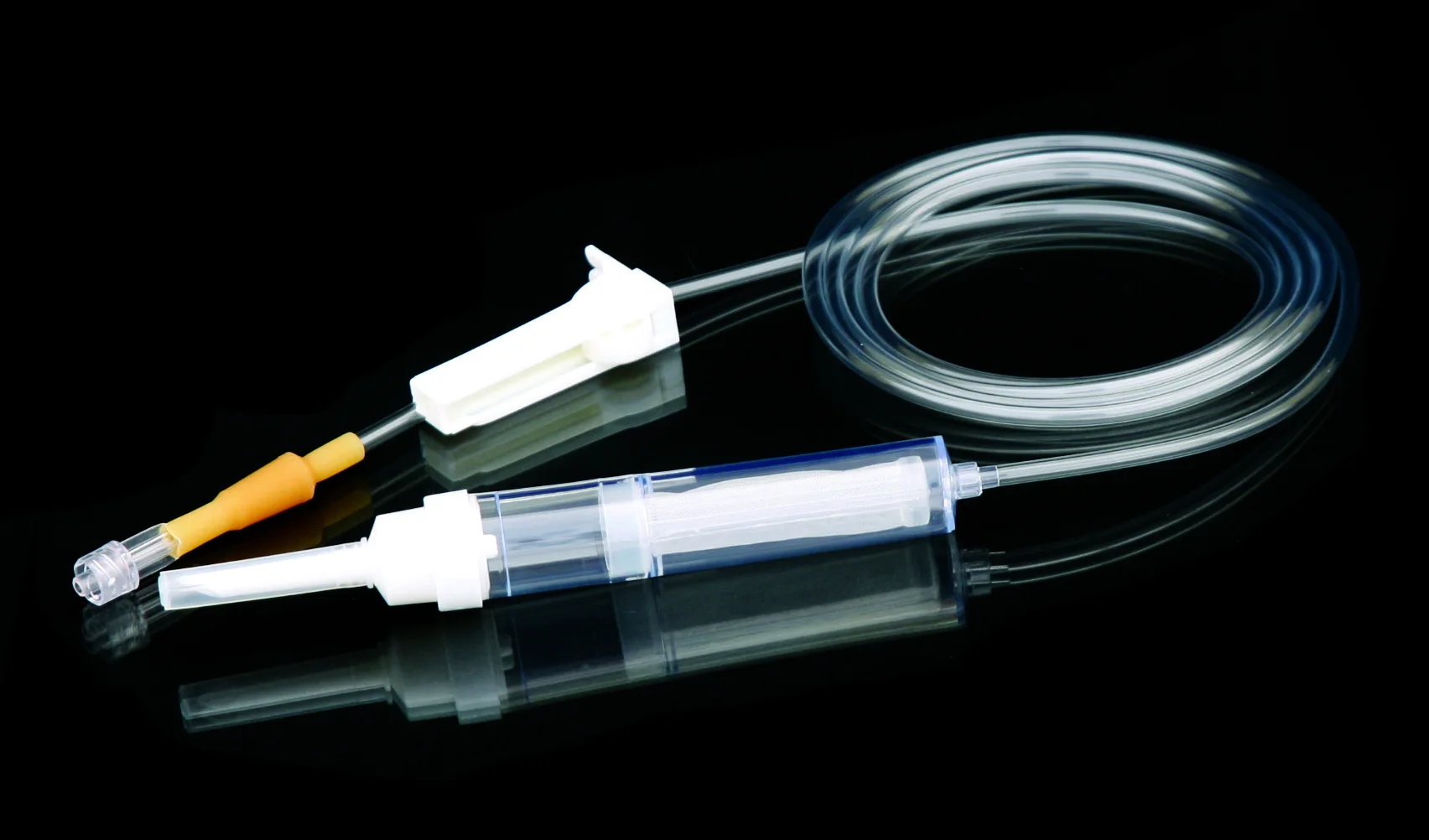
Высококачественные одноразовые наборы для переливания крови без
- Категория: Одноразовые аппарат для переливания крови >>>
- Поставщик: Anhui,Tiankang,Medical,Technology,Co.,Ltd.
Поделиться:
Описание и отзывы
Трекер стоимости
| Месяц | Минимальная цена | Макс. стоимость |
|---|---|---|
| Sep-19-2025 | 0.21 $* | 0.20 $* |
| Aug-19-2025 | 0.17 $* | 0.61 $* |
| Jul-19-2025 | 0.52 $* | 0.70 $* |
| Jun-19-2025 | 0.16 $* | 0.64 $* |
| May-19-2025 | 0.89 $* | 0.12 $* |
| Apr-19-2025 | 0.54 $* | 0.51 $* |
| Mar-19-2025 | 0.39 $* | 0.69 $* |
| Feb-19-2025 | 0.87 $* | 0.23 $* |
| Jan-19-2025 | 0.55 $* | 0.52 $* |
Характеристики
Product Description

Product Usage
Disposable blood transfusion sets are used to save patients with massive bleeding or for blood transfusion during operation.The new model does not contain DEHP. Components are protective spike, drop (containing blood filter), PVC catheter,Y injection port,roller clamp, adaptor, latex, etc.
Product packaging



Foreign Trade Market
Our products sell well in more than 50 countries overseas and regions.
Factory view




Company Profile

Anhui Tiankang Medical Technology Co.,Ltd (Stock Abbreviation: Tiankang Medical, Stock Code: 835942)located in Tianchang City which is rated as the pearl of Anhui.Our company have got official new three board on March 3, 2016 and now we have a registered capital of 57.78 million RMB yuan. We specialize in developing and producing products like safety syringes with retractable needles, auto disable syringes, I.V sets ,T.V sets ,extracorporeal blood circuits for haemodialysers,oral syringes, I.V catheter and intravenous infusion sets with burette etc.
Our company has a factory of more than 600 acres holding a large scaled 100,000 class clean workshop 30,000 square meters.And we now have a staff of one thousand one hundred in including 430 technical engineers of middle and high ranges(about 39% of all the staff). Besides, we now have more than 100 first-class injecting machines and affiliated equipments of assembling and packing. What deserves to be mentioned the most is that we are authorized European CE certificate (in 2002),ISO13485 certificate (in 2002) and WHO—PQS certificate (in 2010) which we hold as passports to enter international market. Our company's products began to be registered with the U.S. Food and Drug Administration in 2012 and currently rank among the best in the country for U.S. Food and Drug Administration-listed products. From June 6 ,2016 to June 9, with zero defect through the U.S. Food and Drug Administration field QSR 820 audit at the U.S. Food and Drug Administration site.
Our company has a factory of more than 600 acres holding a large scaled 100,000 class clean workshop 30,000 square meters.And we now have a staff of one thousand one hundred in including 430 technical engineers of middle and high ranges(about 39% of all the staff). Besides, we now have more than 100 first-class injecting machines and affiliated equipments of assembling and packing. What deserves to be mentioned the most is that we are authorized European CE certificate (in 2002),ISO13485 certificate (in 2002) and WHO—PQS certificate (in 2010) which we hold as passports to enter international market. Our company's products began to be registered with the U.S. Food and Drug Administration in 2012 and currently rank among the best in the country for U.S. Food and Drug Administration-listed products. From June 6 ,2016 to June 9, with zero defect through the U.S. Food and Drug Administration field QSR 820 audit at the U.S. Food and Drug Administration site.
Exhibition

National government leaders visiting




Shippment


Related model



Похожие товары
Одноразовый набор для переливания крови
US $0.10-$1.00
Disposable Blood Transfusion Set
US $0.16
Disposable Infusion Needle Set Butterfly
US $0.023-$0.025