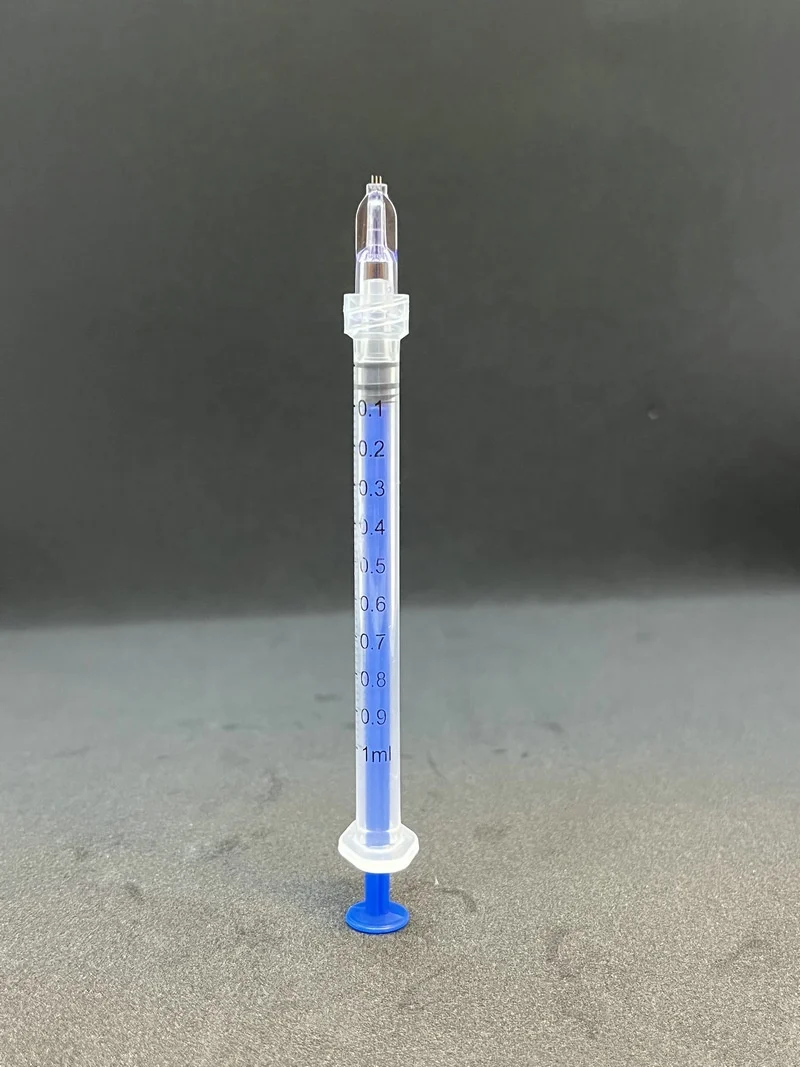
Инъекция иглы 34 г для наполнения NCTF
- Категория: Derma Rolling System >>>
- Поставщик: Zhongshan,Mh,Equipment,Co.,Ltd.
Поделиться:
Описание и отзывы
Трекер стоимости
| Месяц | Минимальная цена | Макс. стоимость |
|---|---|---|
| Sep-18-2025 | 0.91 $* | 0.56 $* |
| Aug-18-2025 | 0.26 $* | 0.66 $* |
| Jul-18-2025 | 0.51 $* | 0.62 $* |
| Jun-18-2025 | 0.26 $* | 0.20 $* |
| May-18-2025 | 0.21 $* | 0.36 $* |
| Apr-18-2025 | 0.2 $* | 0.86 $* |
| Mar-18-2025 | 0.16 $* | 0.6 $* |
| Feb-18-2025 | 0.96 $* | 0.38 $* |
| Jan-18-2025 | 0.26 $* | 0.95 $* |
Характеристики
Nanosoft Microneedle fillmed nctf135 use microneedle nanosoft
There are: 0.6mm,1.0mm,1.2mm,1.5mm nanosoft microneedle choose.
3Pin needle
34G 0.6mm:30pcs/Box
34G1.0mm;34G1.2mm;34G1.5mm:20pcs/Box
Pirce no including 1ml syringe,if you need,feel free message us with cheaper price. All stockage product,can delivery quickly,by Fedex/DHL.
There are: 0.6mm,1.0mm,1.2mm,1.5mm nanosoft microneedle choose.
3Pin needle
34G 0.6mm:30pcs/Box
34G1.0mm;34G1.2mm;34G1.5mm:20pcs/Box
Pirce no including 1ml syringe,if you need,feel free message us with cheaper price. All stockage product,can delivery quickly,by Fedex/DHL.

HONSOFT MICRONEEDLES:
The Honsoft Microneedle is an innovative single use medical device, which enables a nearly painless treatment because of the tiny 34g needle size. The microneedle is made up of 3 silicone micro-cones of 0.6 mm-1.5mm,making the needle three times smaller than a normal needle. Furthermore, the needle improves patient comfort and minimizes the risk of bruising.
The Honsoft Microneedle is an innovative single use medical device, which enables a nearly painless treatment because of the tiny 34g needle size. The microneedle is made up of 3 silicone micro-cones of 0.6 mm-1.5mm,making the needle three times smaller than a normal needle. Furthermore, the needle improves patient comfort and minimizes the risk of bruising.
Product Overviews


Product Show








Related Products
company strength

Honbeauty is a leading supplier of advanced beauty products. Being in this field for 20 years,Honbeauty aims to provide high quality product and service to aesthetic physisian. Spas, Clinics, Doctors and patients worldwide are benifiting from our product and service.


Product Advantage.
1. Zero-risk micro-cosmetic auxiliary consumables
2. The composition of three ultra-thin microneedles is three times that of ordinary needles
3. Ultra-thin needle to reduce the risk of congestion and swelling
Sustained extremely thin penetration up to 400 times
4. Double section tip, small wound mouth, weak pain
5. Unique 3mm double helix veins lock the needle to prevent the needle from disengaging
6. The special blue mark corresponds to the outlet
7. Adopt the interface to prevent the deformation and leakage of the needle in use
8. Imported 304 medical steel has high flexibility and is not easy to break needles
CONTRAINDICATIONS
-The Honsoft microneedle should not be used on skin abrasions, open wounds, cuts and scars.
-The Honsoft microneedle should not be used on rashes, skin infections or any other area of damaged or diseased skin.
WARNINGS AND PRECAUTIONS
-The Honsoft microneedle is intended for single use. by healthcare professionals
-Re-use may lead to infection or other illness/injury.
-Used or un-used devices should not be re-capped or removed from syringe unless there is no alternative or such action is required by a specific medical procedure.
-The Honsoft microneedle is not intended for aspiration of liquids.
-If the device appears to be damaged or broken, discard and use a new device.
ADVERSE EVENTS
Local adverse reactions, including edema, erythema and discoloration of skin at the treatment site have been observed, most likely related to the substance, and not to the Honsoft microneedle.
Adverse reactions related to use of the HONSOFT microneedle include small risk of microbleeding (self-limiting blood dotting,ceasing within normal clotting time). Other potential device related adverse events may rarely include local treatment at the treatment site.
STORAGE AND HANDLING
- HONSOFT microneedle should be stored at room temperature.
-The HONSOFT microneedle is individually packed in a soft blister, sterilized by ethylene oxide [EtO].
-Do not use the HONSOFT microneedle. in the event that the soft blister is either open and/or damaged.
-Dispose of all devices in accordance with national/institutional guidelines.and in their absence, discard in a sharps container.
SUPPLY METHOD
The device is supplied as a single sterile HONSOFT microneedle device, packed in a soft blister for single use.
1. Zero-risk micro-cosmetic auxiliary consumables
2. The composition of three ultra-thin microneedles is three times that of ordinary needles
3. Ultra-thin needle to reduce the risk of congestion and swelling
Sustained extremely thin penetration up to 400 times
4. Double section tip, small wound mouth, weak pain
5. Unique 3mm double helix veins lock the needle to prevent the needle from disengaging
6. The special blue mark corresponds to the outlet
7. Adopt the interface to prevent the deformation and leakage of the needle in use
8. Imported 304 medical steel has high flexibility and is not easy to break needles
CONTRAINDICATIONS
-The Honsoft microneedle should not be used on skin abrasions, open wounds, cuts and scars.
-The Honsoft microneedle should not be used on rashes, skin infections or any other area of damaged or diseased skin.
WARNINGS AND PRECAUTIONS
-The Honsoft microneedle is intended for single use. by healthcare professionals
-Re-use may lead to infection or other illness/injury.
-Used or un-used devices should not be re-capped or removed from syringe unless there is no alternative or such action is required by a specific medical procedure.
-The Honsoft microneedle is not intended for aspiration of liquids.
-If the device appears to be damaged or broken, discard and use a new device.
ADVERSE EVENTS
Local adverse reactions, including edema, erythema and discoloration of skin at the treatment site have been observed, most likely related to the substance, and not to the Honsoft microneedle.
Adverse reactions related to use of the HONSOFT microneedle include small risk of microbleeding (self-limiting blood dotting,ceasing within normal clotting time). Other potential device related adverse events may rarely include local treatment at the treatment site.
STORAGE AND HANDLING
- HONSOFT microneedle should be stored at room temperature.
-The HONSOFT microneedle is individually packed in a soft blister, sterilized by ethylene oxide [EtO].
-Do not use the HONSOFT microneedle. in the event that the soft blister is either open and/or damaged.
-Dispose of all devices in accordance with national/institutional guidelines.and in their absence, discard in a sharps container.
SUPPLY METHOD
The device is supplied as a single sterile HONSOFT microneedle device, packed in a soft blister for single use.